A Tarmogen consists of intact, heat-inactivated yeast containing a target protein. Immunization with a Tarmogen results in antigen-specific cellular immune responses against the target protein and reduction in the number of abnormal cells containing the same target antigen. Tarmogens also reduce the number and function of regulatory T cells, thus further enabling the antigen-specific cellular immune response. Tarmogens target the molecular profile that distinguishes a diseased cell from a normal cell but are not required to be custom manufactured for each individual patient. Tarmogens are manufactured by a process that yields a stable, off-the-shelf product candidate that is disease- or antigen-specific. While some antibody may be generated against the yeast, the antibody does not block the activity of the yeast, allowing for repeated administration and boosting of the immune response with additional administrations. The following graphics and corresponding text describe the mechanism by which we believe Tarmogens work.
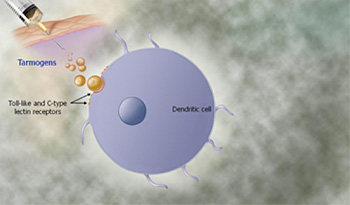 As shown in the graphic to the right, administration of Tarmogens initially results in binding of the yeast to white blood cells called antigen-presenting cells, the most important of which are known as dendritic cells, near the injection site. The dendritic cells are activated as a result of the Tarmogens binding to molecules called Toll-like receptors and other receptor molecules on the surface of the dendritic cell, resulting in the activation of immune signaling molecules called cytokines. The dendritic cell then engulfs the Tarmogen. Multiple Tarmogens may be taken up by the same dendritic cell.
As shown in the graphic to the right, administration of Tarmogens initially results in binding of the yeast to white blood cells called antigen-presenting cells, the most important of which are known as dendritic cells, near the injection site. The dendritic cells are activated as a result of the Tarmogens binding to molecules called Toll-like receptors and other receptor molecules on the surface of the dendritic cell, resulting in the activation of immune signaling molecules called cytokines. The dendritic cell then engulfs the Tarmogen. Multiple Tarmogens may be taken up by the same dendritic cell.
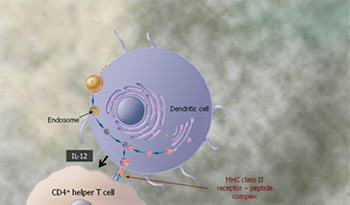 The Tarmogen is processed by the dendritic cell in two ways. First, the Tarmogen is engulfed by subcellular bodies known as endosomes and the protein inside the endosome is cut into shorter fragments called peptides. These peptides are presented by Class II MHC molecules on the surface of the dendritic cell. In combination with IL-12, a cytokine that is produced by the dendritic cell, these MHC-peptide complexes on the surface of the dendritic cell are recognized by and activate cells involved in viral immunity called CD4+ helper T cells.
The Tarmogen is processed by the dendritic cell in two ways. First, the Tarmogen is engulfed by subcellular bodies known as endosomes and the protein inside the endosome is cut into shorter fragments called peptides. These peptides are presented by Class II MHC molecules on the surface of the dendritic cell. In combination with IL-12, a cytokine that is produced by the dendritic cell, these MHC-peptide complexes on the surface of the dendritic cell are recognized by and activate cells involved in viral immunity called CD4+ helper T cells.
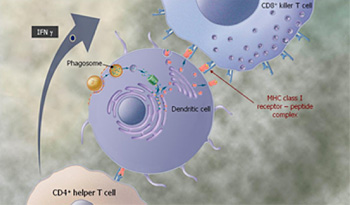 Dendritic cells also process Tarmogens by engulfing them with different subcellular bodies called phagosomes. This results in presentation of peptides, including the antigen from inside the Tarmogen, to cells, known as CD8+ killer T cells, via Class I MHC molecules on the surface of the dendritic cell, resulting in proliferation of identical antigen specific CD8+ T cells. CD4+ helper T cells are so named because one of their roles is to “help” activate killer T cells by expressing a cytokine called interferon gamma, IFNγ.
Dendritic cells also process Tarmogens by engulfing them with different subcellular bodies called phagosomes. This results in presentation of peptides, including the antigen from inside the Tarmogen, to cells, known as CD8+ killer T cells, via Class I MHC molecules on the surface of the dendritic cell, resulting in proliferation of identical antigen specific CD8+ T cells. CD4+ helper T cells are so named because one of their roles is to “help” activate killer T cells by expressing a cytokine called interferon gamma, IFNγ.
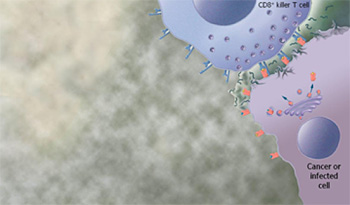 The newly activated CD8+ killer T cells move throughout the body and identify any other cell that expresses the same disease protein as the one recognized by the CD8+ killer T cells. Once the CD8+ killer T cell finds another cell in the body containing the target protein, it can kill the cell using multiple mechanisms.
The newly activated CD8+ killer T cells move throughout the body and identify any other cell that expresses the same disease protein as the one recognized by the CD8+ killer T cells. Once the CD8+ killer T cell finds another cell in the body containing the target protein, it can kill the cell using multiple mechanisms.
In addition to generating these antigen-specific T cell immune responses, Tarmogens also reduce the number and function of regulatory T cells, the component of the immune system that suppresses immune responses of other cells. Regulatory T cells represent an important mechanism built into the immune system to prevent excessive reactions. We believe that suppression of regulatory T cells could further enhance the ability of antigen-specific T cells to eliminate diseased cells.
